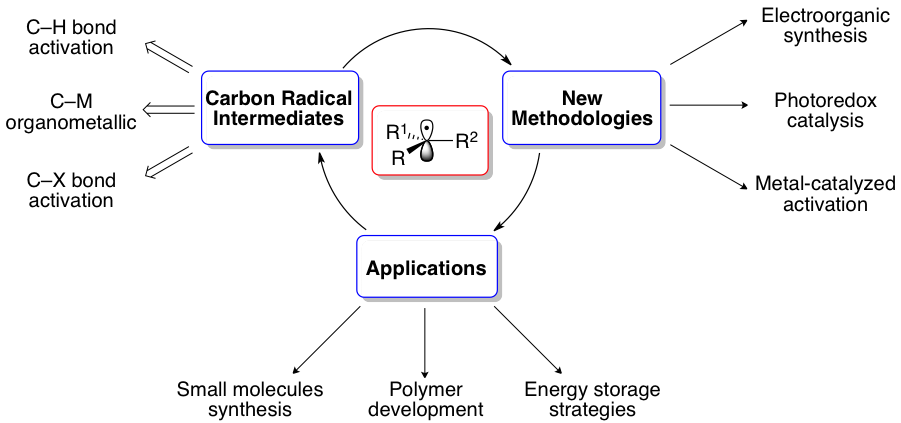
Research in the Laulhé group centers on strategies to induce selectivity in radical-based bond-forming reactions. Our efforts focus on the controlled generation of radicals via photoredox catalysis, organic electrochemistry, and metal-catalyzed technologies. We aim to design catalysts that enable new molecular activation strategies to address unsolved problems in organic synthesis and open the door to novel and non-classical retrosynthetic disconnections.
We aim to apply our novel synthetic technologies to harness the untapped reactivity of readily available starting materials (bench stable organometallic reagents, haloalkanes) and chemical feedstocks (sugars, CO2) to generate highly functionalized value-added products such as pharmaceuticals, biofuels, and materials.
Photoredox catalysis:
Over the past decade, photoredox catalysis has been gaining significant attention in the development of novel synthetic methodologies. In a general sense, excitation with visible light of the photoredox catalysts enables them to engage in single-electrontransfer (SET) processes with organic substrates. This new mode of activation of small molecules generates organic radicals with novel reactivity profiles. Our goal is to understand and exploit these profiles to facilitate carbon–carbon bond formations and functionalization of readily available starting materials.
Asymmetric catalysis:
A main goal in our research is to develop new catalytic strategies for enantioselective functional group interconversions. To do so, we will develop new metal catalysts that enable the generation of open shell intermediates under mild reaction conditions. Importantly, these projects serve as our initial platform for the use of abundant chemical feedstock to generate functionalized high-value products.
Electrosynthesis:
Organic electrosynthesis represents an energy efficient and safe methodology for the oxidation and reduction of organic compounds. Dangerous or unstable reagents can be replaced by electric current or can be generated in situ under mild conditions, rendering the reactions environmentally friendly. We aim to develop new electrosynthetic methodologies for the site-selective functionalization and redox-editing of complex natural products.
INTERESTED IN SEEING MORE?
Get in contact with the team and find out more about what we do!
